PH 14 - pOH. Ad No matter if you look for technical or certified pH buffers we have the solution you need.

Adding Acid To A Buffer Calculating The Ph Using Henderson Hasselbalch Youtube
Calculate the pH of the buffer prepared from a mixture of the salt and weak acidbase.

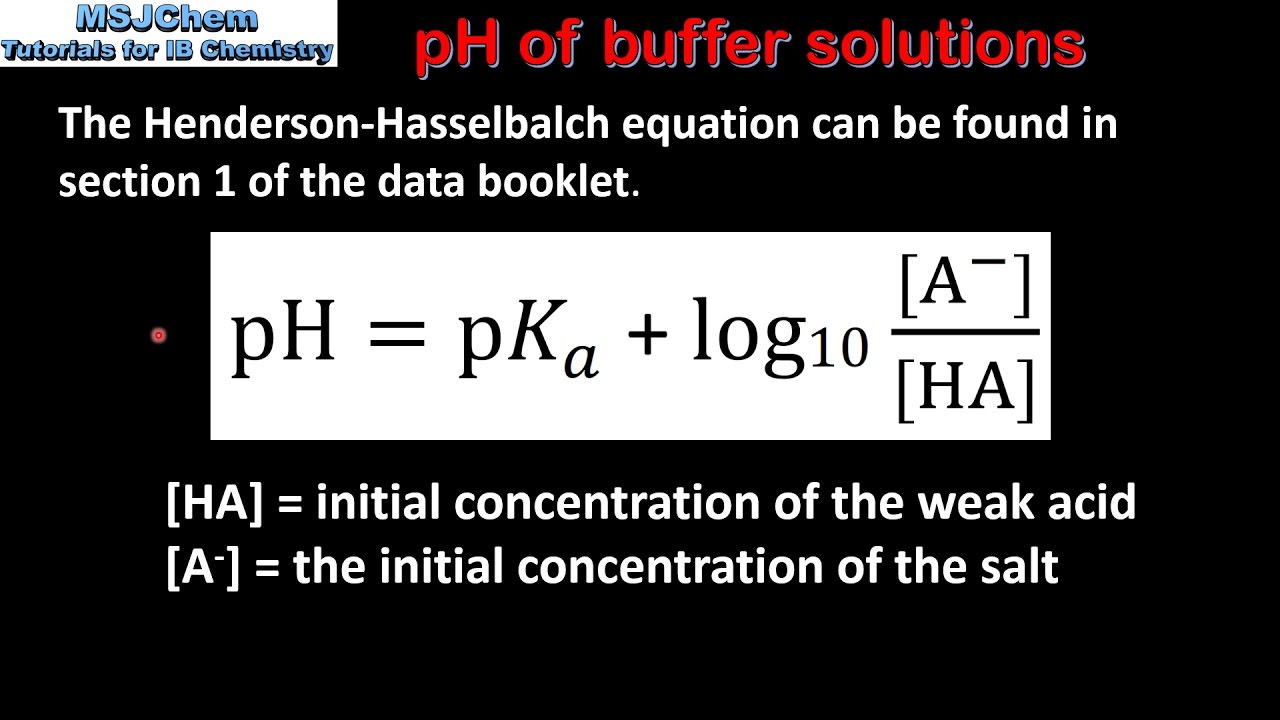
Ph of a basic buffer. PH is equal to the negative logarithm of the concentration of H ions in. Each additional factor-of-10 decrease in the base acid ratio causes the pH to decrease by 1 pH unit. PH pKa log10 A HA Where A denotes the molar concentration of the conjugate base of the acid and HA denotes the.
As such they can absorb excess H ions or OH ions thereby maintaining an overall steady pH in the solution. Optimize your measurements by choosing the right pH buffer solutions. By knowing the Kaof the acid the amount of acid and the amountof conjugate base the pH of the buffer system can be calculated.
Changing the ratio by a factor of 10 changes the pH by 1 unit. Ad No matter if you look for technical or certified pH buffers we have the solution you need. Therefore the pH of the buffer solution is 738.
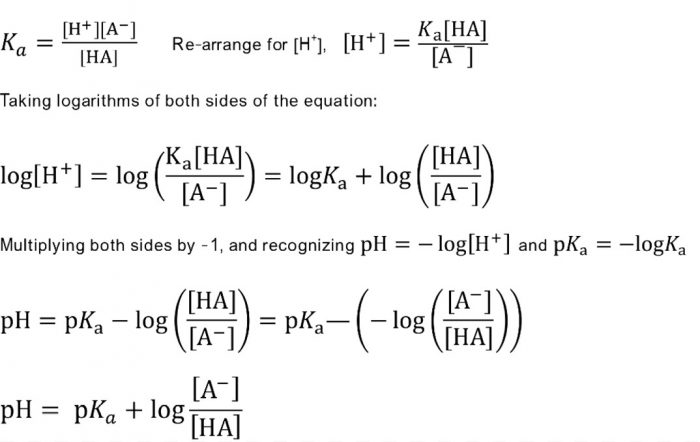
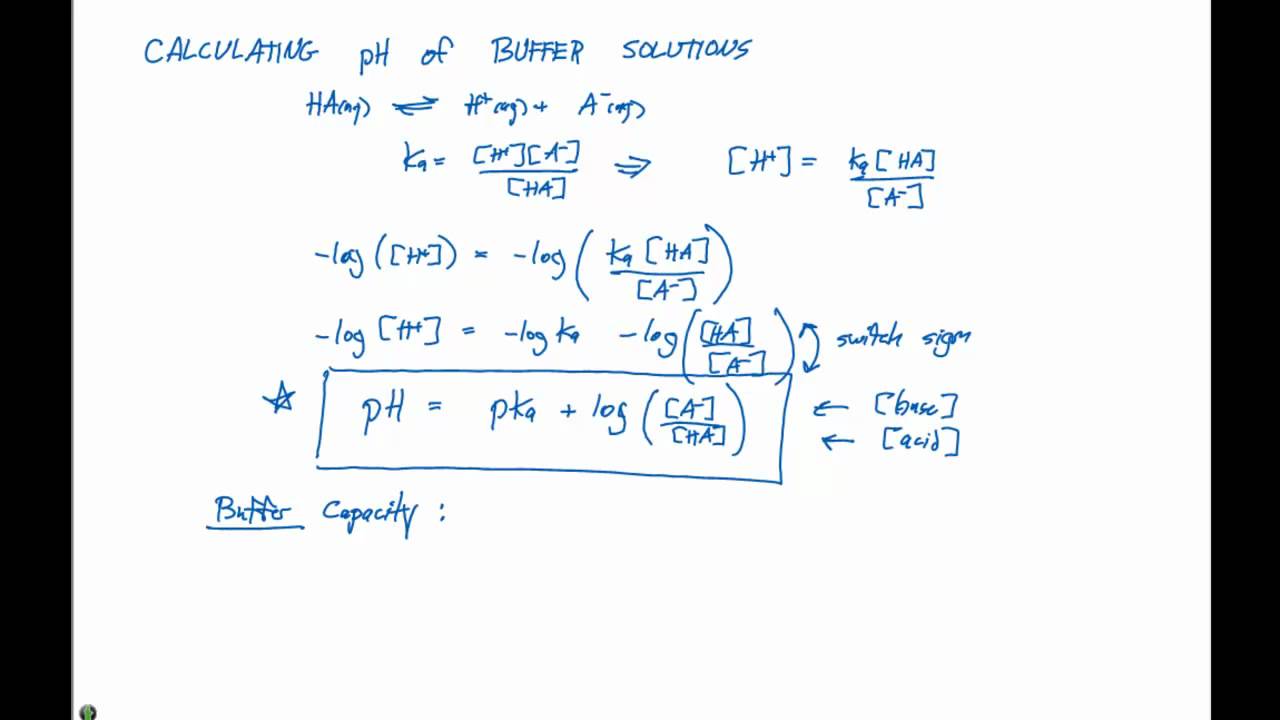
Buffer action of this buffer solution can be explained as follows. Calculation of the pH of a Buffer Solution afterAddition of a Small. Taking negative logarithms of both sides we obtain.
Here we have used the Henderson-Hasselbalch to calculate the pH of buffer solution. POH of a basic buffer pKb log saltacid pH of a basic buffer pKa log saltacid Significance of Handerson Equation. Buffers are solutions that contain a weak acid and its a conjugate base.
This answer is the same one we got using the acid dissociation constant expression. PH 638 1 738. Get a quote now.
7242 log H 3 O log K a log HA A 7243 pH p K a log A HA Equation 7243 is called the Henderson-Hasselbalch equation and is often used by chemists and biologists to calculate the pH of. The Henderson-Hasselbalch equation can be written as. Here the pH is maintained the same due to the neutralization of NH 4 OH by the acid.
Conversely if the base acid ratio is 01 then pH pKa 1. Get a quote now. For More Chemistry Formulas just check out main pahe of Chemsitry Formulas.
H3O KaHA A- pH -logH3O Calculation of the pH of a Buffer Solution. If base concentration of weak base and salt concentration of salt that is mixed to make the buffer. NH 4 OH H 3 O NH 4 2H 2 O.
Handerson Equation can be used to. Optimize your measurements by choosing the right pH buffer solutions. For example a mixture of ammonium hydroxide and ammonium chloride.
PH of a Basic Buffer. If base acid for a buffer then pH pKa.

Acid Base Buffers Calculating The Ph Of A Buffered Solution Video Lesson Transcript Study Com
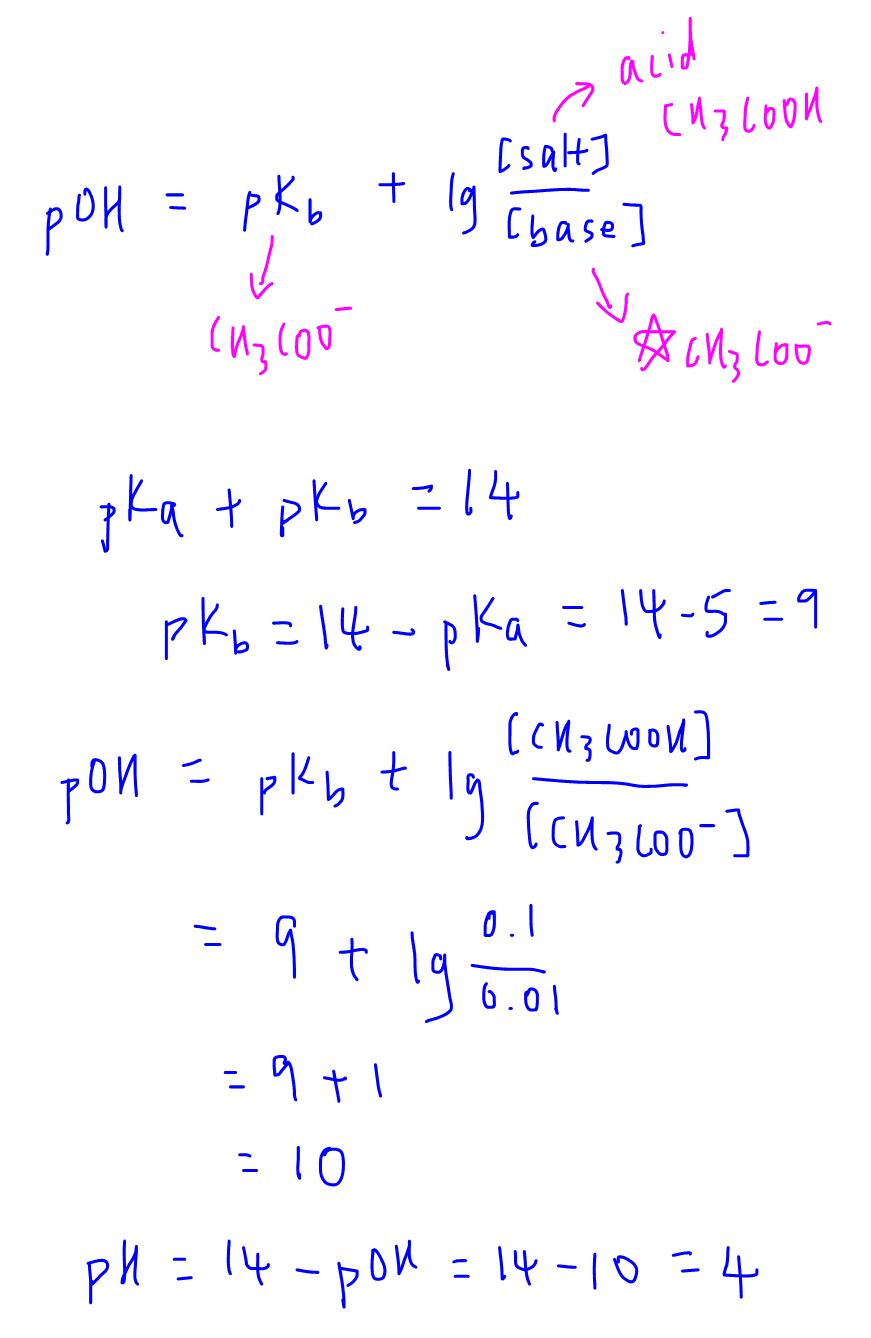
Calculate Ph Of Buffer Solution
Calculation Of Ph Of A Buffer Mixture Chemistry Class 11 Ionic Equilibrium

How To Calculate The Ph Of A Buffer Solution After Adding Acid Hcl Youtube
17 2 Controlling Ph Buffer Solutions Chemistry Libretexts

Calculate Ph Of Buffer After Adding Strong Base Youtube
If The Ph Of A Basic Buffer Solution Is 9 5 What Will Be The Ph When The Concentration Of Salt Present In It Is Increased Four Times
17 2 Calculating Ph Of Buffer Solutions Youtube

Calculation Of Ph Of Acidic And Basic Buffer Solution Henderson Hasselbalch Equation

Buffer Solution Ph Calculations Video Khan Academy

Types Of Buffer Solution In Chemistry

Calculate Ph Of Buffer Solution

The Ph Of Basic Buffer Mixtures Is Given By Ph Pk A Log Base Salt Whereas Ph Of Acidic Buffer Mixtures Is Given By Ph Pk 1 Log Salt Acid Addition Of Little

Buffer Solution Ph Calculations Video Khan Academy

Calculate Ph Of Buffer Solution

Iit Jee Buffer Solution Ph Calculation For Acidic And Basic Buffer Offered By Unacademy
D 4 B 7 Calculating The Ph Of A Buffer Solution Sl Hl Youtube

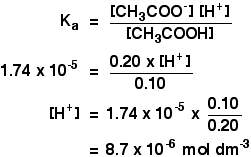

Post a Comment
Post a Comment